With age, human articular and cartilaginous tissues lose their former elasticity, lose their functionality and are destroyed. Such changes can be delayed with the help of a number of drugs. Glucosamine chondroitin is a remedy that helps maintain the functionality of cartilage and joints, relieve symptoms of developing diseases of the musculoskeletal system and improve the quality of life of older people.
What are glucosamine and chondroitin?
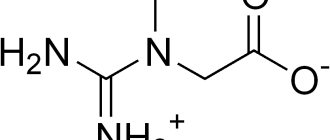
Let's figure out what monosaccharides such as chondroitin and glucosamine are. There is such a thing as chondroitin sulfate. This substance is an essential component of cartilage tissue, which it produces independently. The compound is part of the synovial fluid that fills the joint capsule. These facts indicate that the full functioning of the joints depends on the presence of chondroitin.
Glucosamine acts as a building base for chondroitin and joint fluid. Its deficiency provokes a deficiency of chondroitin sulfate in cartilage tissues. The consequence of this may be the appearance of pain and crunching in the joints.
Types and individual representatives of chondroprotectors
Over the years of existence, chondroprotectors have undergone changes. As a result, there are two generations of drugs from this group on the modern pharmaceutical market. Representatives of the 1st generation chondroprotectors are Alflutop and Rumalon.
For example, Alflutop contains an extract obtained from a mixture of several types of small sea fish. It is successfully produced in Romania in the form of an injection solution, that is, it involves an intravenous or intra-articular route of administration. But at the same time, due to the lack of evidence of effectiveness, it is not on the list of medicines recommended by WHO, is not approved by the FDA, and is not covered in Cochrane reviews.
Following the first generation of drugs, products based on purified glucosamine and chondroitin sulfate appeared, thus forming the 2nd generation of chondroprotectors. Its representatives include:
- Dona, Elbona, Sustilak, Artracam, Sustagard Artro (contain exclusively glucosamine);
- Mucosat, Artra, Structum, Chondrogard (contain only chondroitin sulfate);
- Teraflex, Stopartrit, Honda, Chondro, Chondroglucid (contain both active ingredients).
Subsequently, manufacturers went further and created 3rd generation chondroprotectors. A prominent representative of this group is Teraflex Advance. Its main difference from older products is the inclusion in the composition, in addition to glucosamine and chondroitin sulfate, ibuprofen.
By the way, ibuprofen is a compound belonging to the NSAID group, which has undergone many studies and has significant evidence of pronounced anti-inflammatory, antipyretic and analgesic effects. All children, young parents and older people are familiar with it, since it is the main component of the widely known drug Nurofen.
This is puzzling, since Teraflex Advance really helps to reduce the severity of pain, inflammation and overall improvement of the condition. At the same time, it is not clear: why pay hundreds of times more for cheap ibuprofen?
By analogy with Theraflex Advance, Arthrokera, Arthrodarin, Diarthrin, Diflexa, etc. entered the pharmaceutical market. They contain diacerein, an unpopular representative of the same group of NSAIDs, as an active ingredient. It has good anti-inflammatory and analgesic properties, which makes these drugs effective. But they do not have any direct effect on the condition of the cartilage, its nutrition and protection. Therefore, it is absolutely unclear why they are positioned as chondroprotectors if, in fact, they are drugs of the pharmacological group NSAIDs.
Selected cases: Inoltra and Piascledine
Inoltra in the USA is positioned as a nutraceutical, that is, a kind of food supplement that can have a therapeutic effect on the body. It includes:
- glucosamine and chondroitin sulfate;
- polyunsaturated fatty acids;
- manganese aspartate;
- vitamins C and E.
Since Inoltra is a dietary supplement, it does not need proof of effectiveness and can be freely sold everywhere.
A particularly interesting drug is Piascledine. It is produced in the form of capsules for oral use, and the active ingredients are avocado (100 mg) and soybean oils (200 mg). The price of a package containing 30 capsules is approximately $20.
If you calculate how many oils are contained in all 30 capsules and compare them with the cost of 100 g of each, which is easily sold in every pharmacy, then their total price will be about 340 rubles. Thus, the cost of Piascledine is approximately 60 times higher and it can be replaced without loss of effectiveness with the same oils purchased at the pharmacy. It’s enough just to drop a few drops into a salad, on a piece of bread, sugar, etc.
But again, there is no evidence that avocado and soybean oils improve metabolism in cartilage tissue, help reduce inflammation, eliminate pain, etc.
What is the difference between glucosamine and chondroitin?
One of the questions that people face before using drugs is what is the difference between glucosamine and chondroitin? These substances have the same structure. It is important to know that glucosamine is an essential component of chondroitin, which complements it and enhances its properties. The combination of these substances makes it possible to improve their absorption, provide effective protection and active restoration of cartilage. Most chondoprotectors are drugs consisting only of these components. There are also products in which chondroitin and glucosamine complement the composition.
Properties, benefits and role for the human body
Speaking about why glucosamine chondroitin is taken, it is worth turning to the research of scientists. With long-term therapy with drugs that include this compound, pain relief in gonarthrosis in elderly patients is noted. Their quality of life improves as their joints become more functional. The need for non-steroidal anti-inflammatory drugs in this case is reduced (). The properties of glucosamine chondroitin allow the drugs they contain to perform the following functions:
- ensuring the regeneration of failed and damaged tissues;
- support of the process of cellular nutrition;
- prevention of the formation of age spots;
- fight against inflammatory processes in the gastrointestinal tract;
- preservation of tissue structure at the molecular level;
- preventing the appearance of vascular plaques.
Observations by scientists have led to the conclusion that glucosamine chondroitin is effective as a remedy against osteoarthritis. Its mechanism of action is based on triggering the active production of proteoglycans that have a healthy structure. The benefit of glucosamine chondroitin is that it prevents the destruction of cartilage tissue by inhibiting the activity of matrix metalloproteinase. This compound also helps prevent the spread of inflammatory processes ().
Which chondroprotector is the best?
Only your attending physician can choose the best chondroprotector for your specific case. Our website provides information about the drug Artra®, which is manufactured in the USA under strict quality control. The drug contains high concentrations of glucosamine and chondroitin, which provides a complex effect. The product is available in tablet form for oral administration. The appointment is convenient, only once a day, and this is so important.
- Ilyin, D.P. Diseases of the joints and back in old age / D.P. Ilyin. - M.: Vector, 2011. - p. 100-105
- Maznev, N. Arthritis, arthrosis, gout. Joint diseases. Author's treatment methods / N. Maznev. - M.: Ripol Classic, House. XXI century, 2010. – p. 488-500
- Pokrovsky, Boris Diseases of the joints / Boris Pokrovsky. - M.: Lada, ACC-Center, 2011. - p. 645-648
- Rodionova, O. N. Diseases of the joints / O. N. Rodionova. - M.: Vector, 2012. - p. 245-252
- Worrall, Jennifer Arthritis and other joint diseases. Everything you need to know / Jennifer Worrall. - M.: AST, Astrel, 2015. - p. 124-128
- Badokin V.V., Effective pharmacotherapy, 38/2013. –p.68–75
- Karateev E.A. et al., Use of NSAIDs. Clinical recommendations, IMA-Press, M., 2009. – p. 58-59
- Joint diseases. Guide for doctors / ed. V. I. Mazurova. - 2008. - from 300-305.
Indications for use and instructions for use
According to the instructions for use, glucosamine chondroitin is prescribed in case of diagnosis of osteoarthritis, tendinitis, osteoarthritis and discosis. It is recommended to use drugs with this compound for people prone to injuries, such as dislocations and fractures. Glucosamine chondroitin should be used by people who engage in weightlifting, powerlifting or bodybuilding. Their body is subject to great physical stress, after which it needs help in recovery.
Popular release forms
Glucosamine chondroitin is available in the form of external and internal use. These can be creams, ointments, capsules, tablets and powders. Products for external use do not always give the expected results and do not act as quickly as drugs that need to be taken. Tablets may be slightly less effective than capsules because some of the active ingredient dissolves on the way to the intestines, causing it to lose its benefit. In addition, the tablet form of the drug can cause intestinal irritation. Capsules are more effective and safe. They do not contain additional components that provoke adverse reactions from the gastrointestinal tract, and deliver the active substance intact to the stomach, through the walls of which it is completely absorbed into the bloodstream. Powders are more convenient to use if it is difficult to swallow tablets or capsules.
For what diseases are chondroprotectors used?
Drugs are prescribed for all diseases of the joints. For arthritis (arthrosis) of any etiology and rheumatoid lesions, a preventive drug, for example, a chondroprotector, is useful. Inflammation in these diseases subsequently leads to the destruction of cartilage. Treatment agents are prescribed in a course once every six months, this prevents the destruction of cartilage tissue or restores damage.
If arthritis (arthrosis) is caused by an infectious agent, not only antibacterial agents are needed, but also a drug to protect hyaline, since bacteria, viruses and fungi often cause cartilage destruction.
Treatment takes place in different forms of medications: tablets, injections, ointments. The doctor prescribes the drug and method of delivery.
Intervertebral hernia is treated with chondroprotectors. But what complicates the therapy is that the blood flow in the area of the cartilage of the intervertebral joints is insufficient.
They help with osteochondrosis, especially in the initial stages, when there are no degenerative changes. Medicines are prescribed topically, in the form of ointments or tablets.
For gouty arthritis, you should take a course until the cartilage is destroyed. Since the destruction of cartilage tissue gradually occurs.
In the complex treatment of gonarthrosis (arthrosis of the knee joint), drugs from this group are used.
How to drink glucosamine chondroitin: norm per day, dosage
Glucosamine chondroitin is a drug with a cumulative effect, which is why the duration of its use can be up to 12 months, depending on the clinical picture. Athletes are recommended to drink 1500 mg of the drug in 2 or 3 doses. The maximum daily dose of glucosamine and chondroitin for humans is 2000 mg. The drug is started to be taken in a small amount equal to 400 mg, increasing the dosage according to the dosage regimen. It is drunk during or after meals, since the effect of the active substance slows down on an empty stomach.
What is the problem with modern chondroprotectors?
Even the high market price of chondroprotectors would not pose a big problem if they were very effective. But, unfortunately, they cannot correspond to the declared properties, since glucosamine and chondroitin sulfate entering the bloodstream from the gastrointestinal tract are not able to penetrate from the blood into the joint fluid. In this case, every jelly, jellied meat or aspic would be no less effective.
It is easy to check the distribution pattern of any substance in the body after its administration by any method using a simple radioisotope method. Its essence consists in treating the drug with a radioactive label that is harmless to the human body and has a short duration of existence. By X-ray examination it is possible to determine exactly how it is distributed in the body. If an accumulation of drug particles is detected in the joint area, this will become full proof of its effectiveness.
But pharmaceutical companies are in no hurry to use this method and find many excuses. The reality is that glucosamine is recognized as a dietary supplement in the United States. And independent studies of chondroitin sulfate show that its effectiveness is comparable to the placebo effect.
The only research data that manufacturers of chondroprotectors rely on was obtained by subjective assessment of pain by patients using a visual analogue scale. This cannot confirm the presence of any therapeutic effect from taking chondroprotectors, other than the placebo effect.
It would be possible to talk about their effectiveness if we provide the world medical community with radioisotope research data or show that after taking chondroprotectors, there is at least a minimal increase in the thickness of thinned intervertebral discs.
Contraindications and side effects
Do not take the drug if you are hypersensitive, or if you are pregnant or breastfeeding.
Contraindications to the use of glucosamine chondroitin are also:
- minor age;
- blood clotting disorder;
- diagnosing diabetes mellitus;
- presence of asthma or hypotension;
- disruption of vascular function, including thrombus formation.
When talking about whether glucosamine chondroitin is effective, it is worth taking into account the cumulative effect of the drugs. In order for the therapy to bring results, you need to take the drug for at least 6 months. Thorough research helped determine how safe long-term use of the drugs is. It is noted that effective relief of pain and restoration of joint functionality is observed both while taking the drugs without breaks, and when they are used in courses of 3 months with breaks of the same duration. Side effects of glucosamine chondroitin have been observed in isolated cases. These include abdominal pain after discontinuation of use and a papular rash during use (). In some cases, drugs can cause nausea, vomiting and diarrhea.
Specialists
Blair Yasso1, Yinghe Li2, Asafov Alexander3; Melnikova N.B.4, Mukhina I.V.5
1 MB Research Laboratories, 1765 Wentz Road, Spinnerstown, PA 18968, USA, Blair Yasso, BS, Study Director;
2 Alliance Pharma, Inc., 17 Lee Blvd, Malvern, PA 19355, USA, Yinghe Li, Bioanalytical Principal Investigator;
3 Foura Pacific Pte Ltd, (Singapore), 35 Selegie Road #10-05, Parklane Shopping Mall, Singapore 188307, Asafov Alexander, R&D, CEO;
4 Department of Pharmaceutical Chemistry of the State Budgetary Educational Institution of Higher Professional Education Nizhny Novgorod State Medical Academy of the Ministry of Health of Russia (head of the department - Professor, Doctor of Chemical Sciences Melnikova N.B., Nizhny Novgorod, Rodionova St., 293)
5 Central Research Laboratory of the State Budgetary Educational Institution of Higher Professional Education Nizhny Novgorod State Medical Academy of the Ministry of Health of Russia (Head of the Central Research Laboratory - Professor, Doctor of Biological Sciences Mukhina I.V., Nizhny Novgorod, Gagarin Ave., 70)
Key
words
: relative bioavailability, glucosamine sulfate, transdermal glucosamine complex, micellar transdermal delivery system.
SUMMARY
A comparison was made of the relative bioavailability and intensity of penetration of glucosamine sulfate during oral, injection and local administration of the drug Chondroxide® Maximum in the form of a cream containing a micellar system for transdermal delivery of glucosamine in an experiment on Sprague-Dawley rats. Based on the analysis of the pharmacokinetic profiles of glucosamine in the blood plasma of rats, it was found that when administered daily 3 times a day for a week, Chondroxide® Maximum cream at a dose of 400 mg/kg and a single injection of a 4% solution of Glucosamine sulfate at a dose of 400 mg/kg, the relative bioavailability was 61.6%. The average rate of penetration of glucosamine into the blood plasma through the skin of rats in 4 hours was calculated, equal to 26.9 μg/cm2∙h, and the proportion of penetration of glucosamine from the cream through the skin into the blood plasma of the animal with a single administration in 4 hours, which amounted to 4.12% .
A comparative analysis of literary and experimental data, as well as calculations based on them, allowed us to conclude that, during treatment in accordance with the instructions for the drug Chondroxide® Maximum, the estimated average concentration of glucosamine in the synovial fluid of an inflamed joint can be (0.7– 1.5) µg/ml, which is 10-75 times higher than the concentration of endogenous glucosamine in the synovial fluid of a human joint (0.02-0.07) µg/ml. This value is comparable to the level achieved by injectable forms of glucosamine and is up to 2 times higher than the level achieved by oral forms of glucosamine.
INTRODUCTION
Glucosamine (2-amino-2-deoxy-D-glucose) has reliably proven itself as a chondroprotector in the prevention and treatment of metabolic disorders of bone and connective tissue, in particular in collagen and cartilage matrices. According to the review by R.D. Altman glucosamine as a drug demonstrated symptomatic and osteoarthritis-modifying effects. Glucosamine in this case is a specific substrate and stimulator of the synthesis of endogenous glycosaminoglycans (hyaluronic acid, chondroitin sulfate A and C, dermatan sulfate, keratan sulfate, heparin and heparan sulfate) and, accordingly, proteoglycans, which, along with hyaluronic acid, are one of the main components - building elements of the extracellular matrix connective tissue. The lack of endogenous glucosamine causes inflammatory and degenerative changes, so the development and course of such diseases is greatly influenced by the concentration of glucosamine in the synovial fluid, which can be replenished by introducing glucosamine into the blood plasma using one of the routes discussed here.
Modern research has revealed new additional positive effects from the administration of glucosamine, including normalization of enzyme activity due to the antioxidant protection of superoxide dismutase and catalase, inhibition of the formation of malondialdehyde and diene conjugates - intermediate stages of collagen oxidation, a positive effect on the activity of NO synthase, which generates nitrogen monoxide , with the participation of which glucosamine is synthesized, replenishing the deficiency of sulfate ions necessary for the synthesis of specific glycosaminoglycans.
Glucosamine preparations are widely represented by oral, injection and topical dosage forms containing glucosamine hydrochloride or glucosamine sulfate, suggesting a single dose of 100 to 1500 mg. The reason limiting the use of oral glucosamine preparations in practice is the need to use high concentrations of glucosamine during long-term use to ensure the necessary bioavailability, which can lead to a pro-antioxidant effect. Existing external dosage forms (cream, ointment, emulsion, gel) containing glucosamine do not have an effective mechanism for its transfer to the synovial membrane surrounding the affected area. Therefore, despite the high potential effectiveness of glucosamine, the therapeutic effect of classical local remedies is much inferior to the oral form. Injectable drugs, with their maximum bioavailability, are limited by the method of administration, which is not acceptable for all patients, as well as the need for their use with the participation of professional medical personnel.
One of the ways to improve the bioavailability of glucosamine from external preparations is to introduce a transdermal delivery system for this compound into the dosage form. A representative of such drugs with improved bioavailability is the drug Chondroxide® Maximum, which contains a micellar transdermal glucosamine complex (THC) as a system for the effective delivery of glucosamine into the blood and joint tissues. The achievement of high penetration of THC through the skin is due to the size of micelles of 20-80 nm and the participation in the formation of the micellar system of triglycerides, which enhance the lipophilicity of dispersed phase particles in a direct cream emulsion.
Unfortunately, to date, there have been no pharmacokinetic studies to identify the benefits of using such a transdermal complex and compare its effect with oral and injectable forms, which has hampered the large-scale introduction of this drug into practice.
The purpose of the study
was a comparative experimental study of the relative bioavailability and intensity of penetration of glucosamine sulfate upon its oral and injection administration and local administration of the drug Chondroxide® Maximum THC in an experiment on Sprague-Dawley rats, as well as prediction of the glucosamine content in the synovial fluid of an inflamed human joint.
MATERIALS AND METHODS
Experimental studies were conducted at MB Research Laboratories Spinnerstown, PA (USA) with the assistance of the analytical laboratory Alliance Pharma, Inc., Malvern, PA (USA) in accordance with the Regulations and principles of the current GLP (Good Laboratory Practices) regulations of the Environmental Protection Agency (EPA), 40 CFR Part 160 and 792, Food and Drug Administration (FDA), 21 CFR Part 58, and OECD.
The comparative bioavailability of glucosamine for oral, injection and local administration was studied experimentally using a 4% aqueous solution of Glucosamine sulfate potassium chloride (40 mg of dry powder Glucosamine sulfate/ml) for oral administration and intramuscular administration, distilled water and sterile water for injection were used as the solvent, respectively. . To study external use, the drug Chondroxide® Maximum (Licht Far East (S) Pte. Ltd., Singapore) was applied in the form of a cream containing a system for transdermal delivery of glucosamine in the form of micelles. The composition of the cream (per 100 g) includes: glucosamine sulfate, potassium chloride 8.0 g, excipients, in particular, dimethyl sulfoxide - 1.0 g, ascorbic acid - 0.1 g and formative ingredients. Sprague - Dawley rats weighing 250±25 g ( n =45, each group is 9 - the minimum sufficient number required to be able to take 3 analyzes for each time point (0 hours, 0.5 h, 1 h, 2 h, 4 h, 6 h) and obtain statistically significant data ). At the end of the experiment, the animals were euthanized in a CO2 chamber.
The dose of the study drug Chondroxide® Maximum in the form of a cream for the active substance was 400 mg/kg or for the dosage form 5 g/kg (102 mg/rat). The drug was used externally in the form of a cutaneous application and light rubbing into the back area, from the shoulders to the pelvic bone and down from the back to the abdomen on both sides of the animal, with a total surface of 39 cm2. The application site was left uncovered.
The dose of the studied 4% solution of Glucosamine sulfate potassium chloride for oral administration according to the active substance was 400 mg/kg (88.8 mg/rat). A 4% solution of glucosamine sulfate was administered orally in a volume of 10 ml/kg.
The dose of the studied 4% injection solution was 400, 100 and 25 mg/kg (102.4, 25.9 and 6.2 mg/rat, respectively). The drug was administered intramuscularly in volumes of 10, 2.5 and 0.625 ml/kg of a 4% glucosamine sulfate solution, respectively.
Sample collection and plasma preparation for analysis were carried out according to the following scheme: 0.5 ml of whole blood was collected into tubes containing EDTA as an anticoagulant using direct venipuncture of the rat tail. Blood samples were centrifuged to separate the plasma, which was then divided into two equal parts and placed in two pre-labeled polypropylene cryogenic tubes with screw caps. All collected plasma samples were stored at -70±50C until dispatched via overnight courier (on dry ice) to the Alliance Pharma, Inc. laboratory. (Malvern, PA, USA) to perform the analysis. Analysis of animal blood plasma samples for glucosamine content was carried out using liquid chromatography-tandem mass spectrometry (LC-MS/MS), taking into account the principles described in detail in the article by Liang Zh. et al.. A compatible Sciex API 4000 QTrap mass spectrometer (USA) was used as a detector on a Shimadzu Prominence LC liquid chromatograph (Japan). Chromatograms were analyzed using the Analyst 1.4.2 program. D-Glucosamine Hydrochloride (Toronto Research Chemicals. Inc., Lot number 13-XJZ-183-1, purity 98%) was used as a calibration standard, D-Glucosamine-l,2-IJC2 Hydrochloride (10 μg/ml) was used as an internal standard. , Toronto Research Chemicals, Inc., Lot number 6-SXG-29-1, purity 98%). To construct a calibration graph for glucosamine, a regression method was used, and the lower level for determining the concentration of glucosamine in blood plasma for the developed method was 0.4 μg/ml.
The main pharmacokinetic characteristics were determined from the pharmacokinetic curves: Cmax
– maximum concentration of the drug in the blood;
C4 hours – the average concentration of the drug in the blood during the first 4 hours; tmax
– time to reach maximum concentration;
the
area under the concentration-time kinetic curve . Only AUCt was used to calculate bioavailability.
To assess the bioequivalence of the drug Chondroxide® Maximum in the form of a cream with the active ingredient Glucosamine sulfate, potassium chloride, in comparison with a 4% solution for oral use and a 4% solution for injection of Glucosamine sulfate, a statistical analysis of the obtained pharmacokinetic data (mean value, standard deviation, accuracy) was carried out. using the Watson LIMS™ software for bioanalytical laboratories and in accordance with the Guidelines for Preclinical Drug Studies.
RESEARCH RESULTS
At the first stage, the pharmacokinetic profile of glucosamine in the blood plasma of rats was studied with a single local administration of Chondroxide® Maximum cream (hereinafter referred to as “cream”) on the back area in the amount of 400 mg/kg (Table 1).
A comparison of the injection method of administration with the local one was carried out at three dose levels - 400 mg/kg (102.0 mg/rat), 100 mg/kg (25.9 mg/rat), 25 mg/kg (6.2 mg/rat) , reflecting the range in which the desired effect of glucosamine is realized in rats of a given species without signs of side effects. From the data in Figure 1(B) it is clear that in this dose range the linearity of pharmacokinetics is maintained. The maximum topical Cmax of 7.31 μg/ml is closest to the Cmax of 13.05 mg/ml determined from the pharmacokinetic curve for intramuscular administration at a dose of 25 mg/kg (6.2 mg/rat); AUCt values are 7.35 μg∙h/ml and 10.8 μg∙h/ml, respectively (Table 1).
A comparison of oral (400 mg/kg), intramuscular (25 mg/kg) and local (400 mg/kg) administration is shown in Table 1. From the data in Table 1 it can be seen that the Cmax of glucosamine in blood plasma after intramuscular (IM) administration was achieved after 0.5 hours, with local administration after 1 hour and with oral administration - after 1.5 hours.
Taking into account the above facts, to calculate the relative bioavailability of a cream with a dose of 102.0 mg/rat with a single administration, data on intramuscular administration of a 4% aqueous solution of glucosamine with a dose of 6.2 mg/rat were used.
| A | B |
| IN | |
Figure 1. Plasma concentrations of glucosamine in Sprague - Dawley following different routes of administration. The ordinate is the concentration of glucosamine in µg/ml,
x axis – time of taking a blood sample
Table 1.
Pharmacokinetic parameters of glucosamine in the blood plasma of rats after a single dose
| Location of curves, Fig. 1 (A B C) | A drug | Dose per rat according to dosage form, mg | Pharmacokinetic parameters | |||
| Cmax µg/ml | C4 hours, mcg/ml | AUCt µg∙h/ml | tmax, h | |||
| 1(A), 1(B) | Cream Chondroxide® Maximum (back) | 102,0 | 7,31 | 1,84 | 7,35 | 1,0 |
| 1(B) | 4% solution for oral use | 88,8 | 11,7 | 4,93 | 19,7 | 1,5 |
| 1(B) | 4% solution for injection (10 ml) | 102,4 | 290,7 | 62,6 | 250,4 | 0,5 |
| 1(B), 1(C) | 4% solution for injection (0.625 ml) | 6,2 | 13,05 | 2,7 | 10,8 | 0,5 |
To select a criterion for comparative assessment of local administration in relation to other methods, an approximate calculation of the mass of glucosamine (GA) in rat plasma after a single administration (GGA, mg) was performed using the ratio of AUCtop to AUCx, and the mass of glucosamine in one dose for a certain method of administration of mGA (x):
SGA, mg = (AUCtop/ AUCx)* mGA(x),
where AUCx represents the values:
AUCin,max – AUC upon injection with a maximum dose of 102 mg per 1 rat;
AUCin,min – AUC upon injection with a dose of 6.2 mg per 1 rat;
AUCperos - AUC when administered orally with a dose of 88.8 mg per 1 rat.
table 2
Comparative characteristics of administration methods
| Introduction | Comparison | AUCtop/ AUCх | SGA, mg |
| Locally 400 mg/kg (102 mg) | Injection 400 mg/kg mHA =102 mg | 7,35/250,4 | 3,0 |
| Locally 400 mg/kg (102 mg) | Injection 25 mg/kg mHA =6.2 mg | 7,35/10,8 | 4,2 |
| Locally 400 mg/kg (102 mg) | Oral 400 mg/kg mGA =88.8 mg | 7,35/19,7 | 33,1 |
From the data in Table 2 it follows, for example, that a single application of a cream weighing 1.28 g (dose of glucosamine 102 mg) to a rat corresponds to a single injection of a 4% glucosamine solution in an amount of 4.2 mg.
CALCULATION OF COMPARATIVE BIOAVAILABILITY THROUGH HUMAN SKIN
The penetration of glucosamine from the cream through skin with an area of 39 cm2 into the blood plasma was assessed through the fraction α, % and the specific penetration rate υpr (specific diffusion)
The penetration fraction of glucosamine α (%) of a cream of mass m with a single local injection through the skin into the plasma in 4 hours was equal to:
α, % = (GAS in plasma, mg/ m, mg)*100 = 4.2/102 *100 = 4.12%,
and the specific penetration speed is υpr. glucosamine corresponded to 26.9 μg/cm2/h:
υpr = (SGA (6.2 mg) in plasma, μg/Skin, cm2)/ 4 h = (4200 μg/39 cm2)/4 h = 26.9 μg/cm2/h
The value α,% can be considered as the bioavailability of glucosamine 4 hours after applying the cream.
To predict the penetration of β in vivo glucosamine from the drug Chondroxide® Maximum into human skin, we used the following equation obtained by the authors of the article:
β invivohuman = K′eff * υinvivorat,
where K′eff = υinvitrohuman/υinvitrorat ≈ 0.3-0.5, determined in an experiment in a diffusion chamber using the dialysis method; υinvivorat = 26.9 μg/cm2/h.
In accordance with the above dependence, the specific rate of penetration (diffusion) of glucosamine into the blood plasma when applying the drug Chondroxide® Maximum to human skin was the value β invivohuman = (8.1-13.5) μg/cm2/h.
PREDICTION OF GLUCOSAMINE CONCENTRATION IN SYNOVIAL FLUID
The approximate estimate we obtained for the specific rate of transfer of glucosamine from the cream into the blood plasma of a rat in vivo, equal to 26.9 μg/cm2/h, is comparable to the value of the rate of transdermal transfer from a glucosamine solution to the skin in in vitro experiments in a model diffusion cell υinvitro = 19.2 ± 0 .6 µg/cm2/h. The specific transfer rate under these model conditions of glucosamine in a cream of a different composition with a transdermal delivery system lies in the range from 23.5 ± 2.3 to 48.2 ± 1.9 μg/cm2/h. Considering that in real experimental conditions in vivo on rats υ invivo is almost equal to the average value υ invitro determined in a dialysis cell, we can consider our approximate estimate υ = 26.9 μg/cm2/h as a value characterizing the effective penetration of glucosamine from the cream through the skin into the blood plasma.
A comparison of data from the analysis of the pharmacokinetic profiles of glucosamine for human blood plasma, obtained in our work with a single oral dose of 1500 mg, and rat blood plasma with external application of Chondroxide® Maximum cream in our work, indicates the high bioavailability of glucosamine from the drug under study. Pharmacokinetic parameters – AUCt (µg*h/ml) when administered orally to humans is 3.6±0.72, and when the cream is applied externally to a rat is 7.35±1.48; Cmax, µg/ml is 0.49±0.16 (orally for humans) and 7.31±2.08 (topically, Chondroxide® Maximum cream for rats).
The approximately calculated value of β in vivo in humans can be used to estimate the level of glucosamine concentration in the blood plasma of the microvasculature surrounding the joint. For example, according to the instructions for the drug Chondroxide® Maximum in the form of a cream with THC, the amount of cream applied is a strip of cream up to 3 cm long, which approximately corresponds to 3 - 3.5 g. While maintaining the specific amount of cream applied to the skin used in animal experiments in within 4 hours, the area for applying the cream will be 106 cm². The amount of glucosamine delivered into the blood plasma through the capillaries of the papillary dermis will be: (8.1 – 13.5) µg/cm²/hour * 106 cm² * 4 hours = (3434 – 5724) µg.
Assuming the volume of circulating blood in a person is equal to an average of 5500 ml, the most pessimistic (without taking into account the direct penetration of glucosamine in the THC composition through the skin to the synovial membrane) estimate of the average concentration of glucosamine in the blood plasma of the microvasculature surrounding the joint over 4 hours will be value: (3434 – 5724) µg / 5500 ml = (0.62 – 1.04) µg/ml.
When treating osteoarthritis with an injection drug based on glucosamine (LSR-000050 dated December 26, 2007), the course of treatment is 3 intramuscular injections per week, 3 ml each, with a glucosamine content of 400 mg per injection or 0.072 mg/ml blood plasma.
Bioavailability of injectable glucosamine in a large animal model.
According to the authors, the peak concentration (Cmax) of glucosamine in plasma with a single intravenous administration to a healthy horse at the rate of 0.2 mg per 1 ml of blood plasma reached 50 μg/ml, the average concentration of glucosamine in blood plasma over 12 hours was 4.5 µg/ml, and the peak synovial fluid glucosamine concentration was 1.5 µg/ml with a 12-hour mean synovial fluid glucosamine concentration of 0.7 µg/ml.
It should be noted that when glucosamine penetrates from the blood plasma into the synovial fluid, the level of the maximum concentration of glucosamine in the synovial fluid of the inflamed joint, in accordance with the experimental data obtained, can be up to 5 times higher than in the intact joint. The data presented allow us to believe with a high degree of confidence that with osteoarthritis of various stages, the peak concentration of glucosamine in the synovial fluid of the inflamed joint of a horse when administered intravenously can reach a value of 7 - 8 mcg/ml.
Bioavailability of glucosamine by injection in humans.
Considering that the injection of glucosamine to a person is carried out at a concentration 3 times lower than in the model discussed above (0.072 mg per 1 ml of blood plasma), the peak concentration of glucosamine in the blood plasma during a single injection will be approximately 15 - 20 μg/ml, the average concentration in plasma within 12 hours after injection will be about 1.5 - 1.7 μg/ml, and the average concentration of glucosamine in the synovial fluid of a healthy human joint will be 0.25 - 0.3 μg/ml.
Oral bioavailability of glucosamine in humans.
When taken orally, adults with osteoarthritis are prescribed a maximum dose of 1500 mg of glucosamine per day, which is 0.2 mg/ml of blood plasma. Glucosamine is taken orally in the form of capsules or a prepared aqueous solution. The recommended duration of therapy, depending on the severity of the disease, is from 4 to 12 weeks. According to the authors, when 1500 mg of glucosamine is administered to a person once, the peak concentration of glucosamine in the human blood plasma reaches 1.6 μg/ml by 3 hours after administration, the average area under the concentration-time curve over a period of 48 hours is 14.6 μg* hour/ml, then the average concentration from a single dose of glucosamine at a dose of 1500 mg over 48 hours will be 0.3 μg/ml. Accordingly, a dose of 1500 mg taken every day will give an average concentration in human plasma of no more than 0.6 μg/ml. Later work by the same authors shows that when an oral dose of 1500 mg of glucosamine is administered daily for 14 days, glucosamine accumulates in the blood plasma and synovial fluid of a human joint and on the 14th day the average concentration of glucosamine in the blood plasma increases from the endogenous concentration levels are 0.052 µg/ml to 1.28 µg/ml, in the synovial fluid of the joint, respectively from 0.037 µg/ml to 0.78 µg/ml. In other words, the average concentration of glucosamine in the blood plasma after 14 days of administration increases approximately 20 times compared to the endogenous level of glucosamine concentration and 2 times compared to the level of average glucosamine concentration achieved after a single dose. The average concentration of glucosamine in synovial fluid after 14 days of administration also increases approximately 20 times.
Oral bioavailability of glucosamine in a large animal model.
When glucosamine is administered orally to a horse, once, at a dose of 0.2 mg/ml, the peak concentration in blood plasma is 1.13 μg/ml, the average concentration of glucosamine in blood plasma 12 hours after administration is 0.4 μg/ml , the peak concentration of glucosamine in synovial fluid is 0.16 μg/ml, the average concentration of exogenous glucosamine in synovial fluid is 0.072 μg/ml. Taking into account the fact that the specific doses for oral administration to horses and humans in the cases under consideration are equal (0.2 mg/ml), and the parameters of the peak and average concentrations of glucosamine in the blood plasma are very close, it can be assumed that in humans with a single oral administration of glucosamine the average concentration of glucosamine in the synovial fluid will be the same value of 0.07 μg/ml.
Then we can also draw a general conclusion that follows from all the above experimental data on the bioavailability of glucosamine when administered orally to horses and humans. With daily oral administration of glucosamine for 14 days, glucosamine accumulates in the blood plasma and synovial fluid of the inflamed joint; the average concentration of glucosamine, in comparison with the results obtained with a single administration, increases, respectively, by approximately 2 times in the blood plasma in humans from 0.6 μg/ml to 1.28 μg/ml and approximately 11 times in the synovial fluid of an inflamed human joint from 0.07 μg/ml to 0.78 μg/ml.
THE DISCUSSION OF THE RESULTS
Based on research and theoretical calculations, with a single intramuscular injection of glucosamine in a 4% solution of Glucosamine sulfate potassium chloride to a person at a dose of 400 mg (0.072 mg/ml of blood plasma), the peak concentration of glucosamine in plasma can be estimated at 15 - 20 μg/ml , which provides an average level of glucosamine concentration in the synovial fluid during the first 12 hours of 0.25 - 0.3 μg/ml for a healthy joint, and a value of up to 1.25 -1.5 μg/ml for an inflamed joint with a half-life of about 68 hours. With repeated administration 3 times a week, since the next administration occurs after 48 - 72 hours, the concentration of glucosamine in the synovial fluid before each subsequent administration drops to the level of 0.6 - 1.0 μg / ml, and the average concentration of glucosamine in the synovial fluid of the inflamed joint during the course of treatment can be estimated at 1.2 - 1.3 mcg/ml.
Thus, the average concentration of glucosamine in the synovial fluid of the inflamed joint during the injection course of treatment (3 injections per week, IM, 3 ml with a glucosamine content of 400 mg per injection) can be estimated at 1.2 - 1.3 mcg /ml.
When a patient with osteoarthritis takes 1500 mg of glucosamine orally for at least 14 days, daily, in the form of capsules or solution, the average concentration of glucosamine in human blood plasma will reach at least 1.3 μg/ml, the average concentration in the synovial fluid of an inflamed human joint will be not less than 0.7 - 0.8 μg/ml.
Thus, the average concentration of glucosamine in the synovial fluid of an inflamed joint during an oral course of treatment (orally administered at a dose of 1500 mg of glucosamine per day for 14 days) can be estimated at 0.7 - 0.8 μg/ml.
When applying the drug Chondroxide® Maximum, cream with THC once, the average concentration in the blood plasma within 4 hours after each application of THC will be 0.62 - 1.04 mcg/ml.
There are then two ways to evaluate the effectiveness of a standard course of THC treatment. If we take the dependences obtained for oral administration as a basis for the calculation, we obtain the following values. Since the level of the average concentration of glucosamine in the blood plasma after a single application is very close to the values obtained with a single oral administration (0.6 μg / ml), and the frequency of administration is 3 times higher, then with a high degree of probability the mechanism of accumulation of glucosamine in plasma can be applied blood and synovial fluid of the joint. Then, making the assumption that a 3 times greater frequency of administration will lead to a concentration 3 times greater, we conclude that after a course of treatment with Chondroxide® Maximum, cream with THC for 14 days, the average concentration in the blood plasma can be at least 1.8 – 3.1 µg/ml, and in the synovial fluid of an inflamed joint the value is about 0.9 – 1.5 µg/ml or an average of 1.2 µg/ml.
If we take the dependencies obtained for the injection model as a basis for the calculation, we obtain the following values. The average concentration of glucosamine when applying THC is approximately 1.6 times lower than when injecting it, so the average concentration of glucosamine in synovial fluid will be approximately the same times lower and will be 0.17 μg/ml for a healthy joint. Taking into account the information that the level of maximum concentration of glucosamine in the synovial fluid of an inflamed joint is approximately 5 times higher than in an intact joint, the average concentration in the synovial fluid of an inflamed joint can be estimated at 0.8 - 0.9 μg/ml for a patient joint If we take into account that with each application of THC (approximately every 6-8 hours) the concentration of glucosamine in the synovial fluid of the inflamed joint will be restored to the maximum possible level, then we can conclude that the concentration of glucosamine in the synovial fluid of the inflamed joint in this case will be practically maintained at level 0.7 - 0.8 µg/ml or an average of 0.75 µg/ml.
Thus, the average concentration of glucosamine in the synovial fluid of the inflamed joint during the course of treatment with Chondroxide® Maximum, cream with THC (applying the cream 3 times a day in an amount of 3 -3.5 g for 14 days) can be estimated as (0. 7–1.5) µg/ml.
CONCLUSIONS
1. It was experimentally revealed that when the drug Chondroxide® Maximum, cream with glucosamine at a dose of 400 mg/kg, 3 times a day, daily, for a week, was applied to the skin of Sprague-Dawley rats, the amount of glucosamine introduced into the blood plasma using Transdermal Glucosamine Complex amounted to 61.6% of that obtained with a single injection of a 4% solution of Glucosamine sulfate potassium chloride to an animal at a dose of 400 mg/kg.
2. The average value of the specific diffusion of glucosamine when applying the drug Chondroxide® Maximum, cream with Transdermal Glucosamine Complex to the skin of an animal at a dose of 0.033 g/cm² equal to 26.9 µg/cm²/hour is comparable with literature data on the rate of transdermal transfer in in vitro experiments, which ranges from 19.2±0.6 µg/cm²/hour to 48.2±1.9 µg/cm²/hour.
3. A comparative analysis of literary and experimental data, as well as calculations based on them, allowed us to conclude that during treatment in accordance with the instructions for the drug Chondroxide® Maximum, the estimated average concentration of glucosamine in the synovial fluid of an inflamed joint can be (0.7–1 .5) µg/ml, which is 10-75 times higher than the concentration of endogenous glucosamine in the synovial fluid of a human joint (0.02-0.07) µg/ml. This value is comparable to the level achieved by injectable forms of glucosamine and is up to 2 times higher than the level achieved by oral forms of glucosamine.
Relative bioavailability and penetration of glucosamine after topical treatment by Hondroxid® Maximum transdermal glucosamine complex in comparison with oral, injectable routes in experiment on the rats.
Blair Yasso1, Yinghe Li 2, Asafov Alexander3; Melnikova NB4, Muchina IV5
1MB Research Laboratories, 1765 Wentz Road, Spinnerstown, PA 18968, USA, Blair Yasso, BS, Study Director;
2Alliance Pharma, Inc., 17 Lee Blvd, Malvern, PA 19355, USA, Yinghe Li, Bioanalytical Principal Investigator;
3Foura Pacific Pte Ltd, (Singapore), 35 Selegie Road #10-05, Parklane Shopping Mall
Singapore 188307, Asafov Alexander, R&D, CEO;
4 Department of pharmaceutical chemistry of NSMA Russian Ministry of Health (Head of department – Professor, Dr. Melnikova NB, Nizhny Novgorod, Rodionova str, 293)
5Central Research Laboratory of NSMA Russian Ministry of Health (Head of Central Research Laboratory - Professor, Dr. Mukhina IV, Nizhny Novgorod, Gagarin Avenue, 70)
Key words:
relative bioavailability, glucosamine sulfate, transdermal glucosamine complex, micellar transdermal delivery system
SUMMARY
A comparison of the relative bioavailability and intensity of penetration of glucosamine sulfate in oral, injection and topical administration of the dosage form Hondroxide® Maximum as a cream containing micellar system for transdermal delivery of glucosamine in the experiment by Sprague-Dawley rats was carried out. On the base on the pharmacokinetic profiles data of glucosamine in rat blood plasma with daily administration in 3 times a day for 1 week by cream Hondroxide® Maximum 400 mg/kg and the single injection solution of 4% Glucosamine sulfate 400 mg/kg was found that the relative bioavailability was 61.6%. Calculated penetration rate of glucosamine in the plasma through the rats skin in 4 hours, equal to 26.9 μg/cm2 ∙ h, and the penetration of glucosamine through the skin into the plasma after a single dose of cream in 4 hours was 4.12%.
Comparative analysis of literature and experimental data and calculations based on them suggest that medicine Hondroxide® Maximum, cream with transdermal glucosamine complex in the treatment in accordance with the instructions can provide an average concentration of glucosamine in the synovial fluid of an inflamed joint in the range (0.7-1.5) µg/ml, much higher than the concentration of endogenous glucosamine human synovial joint fluid (0.02-0.07 µg/ml).
By theoretical calculations taking into account experimental data it is shown that the medicine Hondroxide® Maximum can reach the bioavailability level of the modern injection forms and exceed the bioavailability level of modern oral forms of glucosamine up to 2 times.
BIBLIOGRAPHY
1. R.D. Altman, Clinical Pharmacology
,
2
(4), 359-371 (2009)
2. M. L. Tiku, H. Narla, M. Jain, P. Yalamanchili, Arthritis Research & Therapy, 9(4), R76, 1-10 (2007).
3. C. Valvason, E. Musacchio, A. Pozzuoli, et al., Rheumatology, 47, 31-35 (2008).
4. V.V. Beregovykh, N.V. Pyatigorskaya, Yu.A. Prudkevich, S.A. Kedik, Bulletin of MITHT,
7
(5), 17-22 (2012).
5. A. Sharma and S. Arora, World Journal of Pharmacy and Pharmaceutical Sciences, 2(6), 6448-6462 (2013).
6. Z. Liang, J. Leslie, A. Adebowale, et al., Journal of Pharmaceutical and Biomedical Analysis, 20(5), 807-814 (1999).
7. Guidelines for conducting preclinical studies of drugs
. Part 1, Grif i K, Moscow (2012).
8. B. Van Ravenzwaay and E. Leibold, Human & Experimental Toxicology, 23, 421-430 (2004).
9. C. G. Jackson, A. H. Plaas, J. D. Sandy, et al., Osteoarthritis and Cartilage, 18, 297-302 (2009).
10. M. Meulyzer, P. Vachon, F. Beaudry, et al, Osteoarthritis Cartilage, 16(9), 973-979 (2008)
11. M. Meulyzer, P. Vachon, F. Beaudry, et al, Osteoarthritis Cartilage, 17(2), 228-34 (2009)
12. S. Persiani E. Roda, L. C. Rovati, et al, Osteoarthritis Cartilage, 13(12), 1041-9 (2005)
13. S. Persiani, R. Rotini, G. Trisolino, et al, Osteoarthritis Cartilage, 15(7), 764-72 (2007)