A woman's hormonal system is fragile. There are many reasons for hormonal imbalance. It is not always possible to quickly find the cause of the failure, because several organs and systems are responsible for the correct balance of hormones in a woman’s body:
- central nervous system via the hypothalamus and pituitary gland;
- thyroid;
- adrenal glands;
- pancreas.
Depending on where exactly the failure occurred, the symptoms differ. But since we are talking about women's health, we will consider the symptoms of hormonal imbalance, in which the balance of sex hormones produced by the ovaries is disrupted.
Symptoms of female sex hormone imbalance
Sex hormones determine a woman’s appearance, her voice, behavior, and are responsible for reproductive function. The most important female hormone, estrogen, is responsible for the soft contours of the body, large mammary glands, wide hips, and a high-pitched voice. Testosterone is also present in the female body, but in less quantity than in men. The female body produces progesterones, prolactin, gonadotropin hormone, and gonadotropin-releasing hormone.
The imbalance is often visible to the naked eye. Here are typical symptoms of hormonal imbalance in women:
- a figure formed according to the male type;
- excess weight, a particularly typical picture is when the stomach is much larger than the chest, and the buttocks are flat (may be a symptom of PCOS);
- hair growth, as in men, for example, hair growth on the chest (single hairs on the nipples do not apply here), excessive hair growth on the stomach, thighs, and face;
- low rough voice.
Acne also indicates that there is a lot of free testosterone in the blood, but this does not always indicate a hormonal imbalance; this is a common situation during puberty.
Most often, the following signs indicate that something is wrong with hormones in a woman’s body:
- menstrual irregularities - too long or very short interval between menstruation, absence of menstruation, heavy blood loss, scanty spotting;
- lack of ovulation, which can be seen on ultrasound;
- early signs of menopause;
- very pronounced premenstrual syndrome - pain in the mammary glands, headaches, nausea, bloating, mood swings;
- infertility;
- miscarriages;
- decreased libido.
If a woman has nipple discharge, this may indicate that she has elevated prolactin due to prolactinoma, a benign tumor of the pituitary gland. Male pattern hair growth, infrequent menstruation, excess weight, and infertility may be signs of polycystic ovaries.
Estradiol is normal in women and men
For the hormone estradiol, the norm in men ranges from 15 to 71 pg/ml. For the hormone estradiol, the norm in women varies depending on the phase of the cycle. When the cycle begins, hormone production is activated. Estradiol becomes elevated before ovulation and, when the concentration reaches its maximum, ovulation occurs. After the follicle ruptures and the egg is released, the norm for estradiol in women again corresponds to the beginning of the cycle.
Thus, in the follicular phase, estradiol can be increased to 227 pg/ml. In the preovulation phase, estradiol in women ranges from 127 to 476 pg/ml. Finally, the luteinizing phase of the cycle again returns estradiol levels to the range from 77 to 227 pg/ml. If estradiol is elevated after ovulation and remains this way, it can be assumed that pregnancy has occurred.
The estradiol norm in women during menopause decreases significantly and amounts to 19.7 – 82 pg/ml.
We must not forget about fluctuations in hormone levels throughout the day. Estradiol is reduced between 24 and 2 hours. Estradiol is usually elevated between 15 and 18 hours.
General signs of hormonal disorders
In addition to specific symptoms of hormonal imbalance, there are also less typical ones, which may, however, indicate incorrect functioning of the thyroid gland, or be part of the picture of an imbalance of sex hormones:
- decreased or sharp increase in libido;
- excess weight without diet errors;
- fatigue, loss of strength, tearfulness;
- changes in facial features (bulging eyes with hypothyroidism);
- drowsiness, tachycardia, sweating;
- muscle weakness.
Hormonal imbalance in women is characterized by a pattern in which several alarming signs appear simultaneously. The sexual sphere, appearance, and activity suffer.
Treatment of hormonal imbalance is necessary to restore the normal functioning of a woman’s reproductive system, make conception and pregnancy possible, achieve regular ovulation and simply give a woman a high quality of life. The Dr.AkNer clinic will definitely help you understand the causes of the failure and eliminate them.
Many women approaching menopause notice unexplained weight gain and an increase in fat tissue, especially in the abdominal area, despite all efforts to lose weight. Moreover, efforts aimed at weight loss, which previously “worked” successfully, often turn out to be ineffective. In clinical practice, an increase in the volume of adipose tissue in the abdominal area is often the only complaint of perimenopausal women. Most of them perceive a weight gain of 5–10 kg as a mandatory manifestation of adolescence. Weight gain is especially acute for women for whom their physical fitness is associated with their profession: athletes, ballerinas, dancers. As you know, women in these professions have spent years getting used to dieting, monitoring their weight and level of physical activity. That is why they are surprised by weight gain despite an unchanged lifestyle that has been formed over the years.
An explanation of the phenomenon of weight gain during menopause has become possible in the last 10–15 years after a number of large studies.
Classification and clinical significance of weight gain in women
According to statistics, obesity is more common in women than in men. Moreover, with age, the incidence of obesity in them progresses, especially after menopause [1, 2].
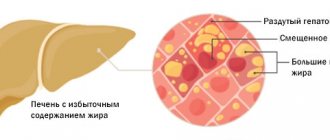
By definition, obesity is an excess of adipose tissue, or more precisely triglycerides in fat cells - adipocytes. At the same time, their volume increases, although the formation of new adipocytes upon stimulation with various substances, in particular glucocorticoids, is not excluded.
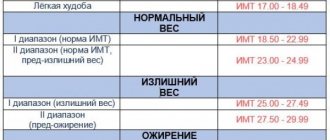
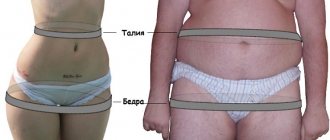
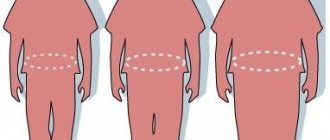
The presence of obesity is determined by body mass index (BMI) - the ratio of body weight in kilograms to height in meters squared. According to the WHO classification, a BMI > 25 kg/m2 indicates overweight, and a BMI ≥ 30 kg/m2 indicates obesity. BMI does not provide information about the amount and distribution of adipose tissue. To obtain a complete picture of obesity, X-ray densitometry and magnetic resonance imaging are used. In clinical practice, the simplest and most common method is to calculate the ratio of waist circumference (WC) to hip circumference (HC) - WC/HR. When WC/TB > 0.8, android (abdominal) obesity is classified, when fat accumulates mainly in the anterior abdominal wall. There are subcutaneous-abdominal and visceral obesity (excess fat in the visceral-mesenteric region). When WC/OB <0.7, obesity is called gynoid and is characterized by excess fat on the thighs [3].
The pattern of fat distribution in women is determined mainly by hormones of the ovaries and adrenal cortex. A risk factor for cardiovascular diseases is abdominal and especially visceral obesity, which is due to the specific anatomical and physiological properties of adipose tissue in such localizations. It has better blood supply and is metabolically more active. Adipocytes are characterized by a high density of beta-adrenergic receptors, the stimulation of which leads to lipolysis, with a relatively low density of alpha-adrenergic receptors and insulin receptors, the stimulation of which lipolysis suppresses [3]. Adipose tissue in the thighs and buttocks is regulated mainly by the enzyme lipoprotein lipase. Here the processes of lipogenesis mainly take place, and the activity of lipolysis is low. In this regard, gynoid obesity does not affect health and only affects the appearance of a woman [4].
Obesity increases the risk of two categories of diseases. The first includes diseases that occur when metabolism is disrupted due to excess abdominal fat: cardiovascular diseases, diabetes mellitus, arterial hypertension, colorectal cancer, breast cancer, liver pathology. Thus, according to epidemiological data, in the United States, among women with a BMI > 30 kg/m2, the incidence of arterial hypertension is 32%, and with a BMI < 25 kg/m2 – 16%; Moreover, an increase in body weight by 10 kg leads to an increase in systolic blood pressure by 3, and diastolic by 2.3 mm Hg. Art. [3–5]. With a BMI from 24 to 24.9 kg/m2, the risk of diabetes in women is 5 times higher, and with a BMI of 31 kg/m2 – 40 times higher compared to patients with normal body weight [6].
According to the results of the Nurses Health Study, with a BMI of 25–29 kg/m2, the risk of coronary heart disease is two times higher, and with a BMI > 29 kg/m2, the risk is three times higher compared to patients with a BMI < 21 kg/m2 . In addition, as BMI increases, the incidence of breast cancer after menopause also increases. In severely obese women (BMI > 45 kg/m2), the incidence of gallstone disease is seven times higher than in patients with normal body weight. It is believed that the formation of gallstones in obesity is due to increased secretion of cholesterol with bile and decreased contractile activity of the gallbladder walls [5].
Diseases of the second category are consequences of obesity as such: osteoarthritis, obstructive sleep apnea (snoring), psychological disorders. It is estimated that with a one kilogram increase in body weight, the risk of osteoarthritis of the knee and metacarpal joints increases by 9–13%. Obstructive sleep apnea affects 60–70% of people with obesity, especially abdominal obesity. Clinically, obstructive sleep apnea may be associated with increased daytime sleepiness, cardiac arrhythmia, myocardial ischemia, hyperventilation syndrome, pulmonary hypertension, heart failure, and stroke.
Many researchers pay attention to the well-known fact that losing body weight significantly reduces the risk of obesity-related diseases. Thus, losing 10 kg of weight leads to a reduction in the risk of arterial hypertension by 26%, with a loss of 20 kg the risk of diabetes mellitus decreases by 87%, and with a decrease in body weight by 5 kg over 10 years, the chance of developing osteoarthritis of the knee joint decreases by 50% [5 ].
Mechanisms of weight gain during menopause
Published information on changes in body weight and adipose tissue distribution after ovarian failure is sparse. Rapid weight gain during perimenopause occurs in approximately 60% of women. According to the Study of Healthy Women, in the first three years after menopause, body weight increases on average by 2.3 kg (in 20% of women - by 4.5 kg or more), and after 8 years - by 5.5 kg [6] . According to most scientists, weight gain is a consequence of menopause without taking into account the influence of age-related changes.
The reasons for weight gain after menopause are not precisely established, although several possible pathogenetic mechanisms are considered. It has been shown that against the background of a decrease in the secretion of ovarian hormones, the total amount of fat increases and is redistributed to the anterior abdominal wall, and the mass of muscle and bone tissue decreases. Several studies have shown that changes in the components of body weight after menopause—decrease in bone density, decrease in muscle mass, and increase in fat mass—occur independently of changes in overall body weight. Densitometry has demonstrated that peri- and postmenopausal women have 8–9% more total fat (especially on the trunk), and less fat and muscle mass on the thighs than premenopausal women [7]. In women of reproductive age who were treated with gonadotropin releasing hormone agonists, the same changes were noted as after natural menopause: an increase in the amount of fat against the background of a decrease in bone and muscle mass [8]. An increase in body weight and the formation of abdominal obesity against the background of a deficiency of sex hormones is explained by changes in the energy balance and regulation of fat cells, enhanced glucocorticoid stimulation, relative hyperandrogenism and other mechanisms.
Weight gain is primarily the result of an imbalance between dietary energy intake and daily energy expenditure for basal metabolism, thermogenesis and physical activity (see figure). It has been established that 65–70% of daily energy expenditure is spent on basal metabolism [9]. There is a strict relationship between the total daily energy expenditure, the proportion of basal metabolism and the mass of muscle tissue, where it is mainly carried out. It has been established that the rate of energy expenditure is inherited, so congenital low metabolic rate may predetermine the possibility of developing obesity.
Thermogenesis, or the thermic effect of food, is the second component (about 10%) of daily energy expenditure. In thermogenesis, there is an obligate function, which cannot be suppressed by drugs, and a facultative function, blocked by beta-adrenergic substances. The latter proves the important role of the sympathetic nervous system in regulating energy balance.
On average, about 25% of daily energy expenditure is spent on physical activity, and this proportion is most variable: from 10% in the weak and elderly to 40–50% in well-trained, active women. That is why the regulation of body weight in postmenopausal women largely depends on physical activity [10].
Since the majority of daily energy expenditure comes from basal metabolism, even small changes in basal metabolism have a significant impact on body weight. Thus, in women of reproductive age in the luteal phase of the menstrual cycle, against the background of high concentrations of progesterone and relatively low estradiol, energy expenditure, body weight, the total amount of food consumed and the fat content in it increase [11].
After menopause, the metabolic rate “at rest” slows down, which is confirmed by the results of a study in which, during dynamic observation, a constant basal metabolic rate was recorded in healthy women under 48 years of age, and in the older age group a significant weakening of metabolic processes was noted (by 4–5 % in each decade of life). It has been established that this degree of decrease in basal metabolic rate after menopause is equivalent to an increase in body weight of 3–4 kg [12].
With long-term observation of women of different ages, it was found that in postmenopause, the resting metabolic rate decreases by approximately 420 kJ/day, while this effect is not observed during reproductive age. Due to the metabolic consequences of a long period of impaired regulation of energy balance, not only does the mass of adipose tissue increase, but the amount of muscle tissue decreases. In particular, using X-ray densitometry, it was found that the mass of muscle tissue after menopause decreases by approximately 3 kg [12].
The mechanisms of the influence of sex hormones on adipose tissue have not yet been fully established. It is believed that the level of sex steroids in the blood determines the nature of the distribution of adipose tissue, since accumulation, intense aromatization of sex hormones and their secretion occur in it. Over the past decades, it has been established that steroid hormones exert their action through the regulation of gene expression by binding to specific receptors in target tissue cells. Currently, two types of estrogen receptors are known: alpha and b. Beta receptors are expressed in adipose tissue [13].
One of the main mechanisms of the influence of sex hormones on adipose tissue is a direct effect on the activity of lipoprotein lipase, the main enzyme regulating the accumulation of triglycerides in adipocytes. In women of reproductive age, lipoprotein lipase stimulates estrogens in the adipose tissue of the thighs and buttocks, where the activity of this enzyme is higher than in the subcutaneous fat of the abdominal region. As a result, lipids accumulate to ensure adequate energy reserves during pregnancy and lactation. After menopause, lipoprotein lipase activity decreases, and adipocytes of the femoral-gluteal region decrease in size, i.e., a relative redistribution of fat occurs [14].
Progesterone is also involved in the regulation of adipose tissue. It competes with glucocorticoids for their receptors in adipocytes, thereby preventing the effects of glucocorticoids on adipose tissue in the late luteal phase of the cycle. The lack of this effect of progesterone partly explains the slowdown in metabolism after menopause. Against the background of a deficiency of sex hormones, lipoprotein lipase is not stimulated and adipocytes of adipose tissue of the femoral-gluteal region no longer serve as a source of energy reserves in the body [14].
The effect of sex hormones on adipose tissue is manifested by the distribution of fat at different periods of a woman’s life. As noted above, reproductive age is characterized by higher lipoprotein lipase activity in the thighs and buttocks, low lipolysis activity and gynoid fat distribution. In postmenopause, lipoprotein lipase activity in this area is low or absent, and intense lipolysis occurs in the subcutaneous abdominal and especially visceral adipose tissue. During hormone replacement therapy (HRT), lipoprotein lipase activity is stimulated in adipocytes of the thighs and buttocks, and in abdominal adipose tissue, enzyme activity is at a low level [15].
Clinical studies have shown that in women with a regular menstrual cycle, appetite depends on the level of estradiol in the blood. In particular, in the periovulatory period, the total amount of food consumed sharply decreases, and in the luteal phase, appetite increases. The presence of a relationship between estradiol levels and dietary patterns in women is evidenced by a decrease in appetite one to two days after an increase in estradiol secretion [16].
No specific region of the brain where estradiol acts as an appetite suppressant has been identified. It is believed that this occurs via a feedback mechanism (as in the interaction with luteinizing hormone) with simultaneous effects on several areas of the brain. Based on the fact of appetite suppression in animals after implantation of estradiol into the hypothalamus, a hypothesis has been proposed that estradiol acts on the ventromedial region of the hypothalamus, thereby suppressing appetite [16].
Estrogens can affect adipose tissue by interacting with leptin, a protein hormone secreted by fat cells. The main function of leptin is to signal to the brain that the body’s energy reserves are sufficient (the saturation threshold). Leptin interacts with specific receptors that are present in various organs and affects the expression of key hypothalamic peptides in the arcuate nucleus, in particular, it reduces the content of neuropeptide Y [16].
A strict relationship has been established between the content of leptin in the blood serum and the amount of fat in the body. When energy is balanced, leptin levels reflect the amount of triglycerides in adipose tissue. When energy intake and expenditure are unbalanced (hunger, overeating), leptin can act as a kind of energy balance indicator. It has been found that with a loss of 10% of body weight, leptin levels decrease by 53%, and an increase in body weight by 10% leads to an increase in leptin levels by 300% [16].
Estrogens regulate leptin production through a positive feedback mechanism. Results from rodent experiments and human tissue studies have shown that leptin receptors are expressed in the ovaries, and estradiol regulates leptin production by adipocytes. Leptin concentrations increase during the luteal phase of the menstrual cycle. Several studies have reported higher leptin concentrations in women of reproductive age compared with levels in postmenopausal women [17].
It is well known that in depression, dysphoria and chronic stress, constant stimulation of the hypothalamus leads to increased activity of the system: corticotropic releasing hormone – adrenocorticotropic hormone – adrenal glands. It is believed that postmenopause also increases glucocorticoid stimulation, which leads to an increase in the size of adipocytes and the formation of abdominal obesity.
Testosterone levels in the blood are directly related to the amount of abdominal fat. In postmenopause, the content of sex steroid-binding globulin decreases, which leads to an increase in the concentration of free testosterone, hyperandrogenism and makes an additional contribution to the formation of abdominal obesity.
Effect of hormone replacement therapy on body weight
Evidence regarding the effects of HRT on weight in postmenopausal women is inconsistent, and prospective studies are limited. For example, Gambacciani M. et al. [17] noted an increase in body weight by 1.9 kg per year in 12 women in the control group (without HRT) and stable body weight in the group receiving HRT. In several studies, patients taking HRT showed a lower WC/TB ratio. In the Nurse Study, in which BMI was taken into account by the participants themselves, a decrease in this indicator was found with the use of estrogens. Several studies have shown that estrogen monotherapy prevents the increase in body fat mass [18].
Thus, according to published data, sex hormones play an important role in the regulation of body weight in women: they act on appetite, daily energy balance and metabolic processes in adipose tissue. Most scientists believe that against the background of a deficiency of sex hormones, regardless of age, an increase in body weight occurs due to an increase in the amount of fat (with the formation of abdominal obesity), a decrease in muscle and bone mass. The results of several studies have shown that estrogen monotherapy and combined HRT restore the gynoid type of adipose tissue distribution characteristic of reproductive age and promote weight loss.
Since 1997, we have examined women who applied to the specialized center “Women's Health after 40 Years.” Since many factors influence body weight in women, we carefully observed one of the conditions for inclusion in the study: the absence of significant fluctuations in body weight before menopause. Thus, our first study on body weight during menopause included 55 women with a weight gain of 5 kg or more that coincided with menopause. As it turns out, many women begin to gain weight during perimenopause. During the initial examination of patients, we found that after menopause, body weight increased by an average of 9.7 kg (5–24 kg) and significantly correlated with the duration of the postmenopausal period. In addition, it turned out that in women with a postmenopausal duration of more than five years, weight gain was significantly greater than in perimenopause. Finally, we found that in postmenopause, body weight and indicators characterizing obesity and its type (BMI and WC) are significantly higher than in perimenopause.
After the baseline examination, 45 women were prescribed HRT. According to our data, the vast majority of them (96.1%) experienced a progressive decrease in body weight by an average of 4.5 kg over the course of a year. The degree of abdominal obesity decreased in 98% of women. A more pronounced and rapid effect was observed in perimenopausal patients: body weight and WC significantly decreased by the sixth month of therapy. A decrease in BMI almost to the upper limit of normal (25.3 kg/m2) was recorded only in the group of perimenopausal women.
We also examined 46 postmenopausal women (mean age 51.2 years, postmenopausal duration 3.8 years). At the time of the examination, 23 patients had been taking the cyclic combination drug Femoston 2/10 (estradiol 2 mg ± dydrogesterone 10 mg) for two years, the remaining 23 women had never taken HRT before. All study participants underwent an anthropometric examination: measurement of height, body weight, WC and BC, calculation of BMI and WC/WC ratio.
The women examined were comparable in age, duration of postmenopause and height. Significant differences between the first and second groups were revealed in body weight, BMI, WC and WC/TB ratio. In the group of patients taking Femoston 2/10, there were significantly more women with normal body weight (BMI 18–25 kg/m2) than in the group without HRT: 34.8 versus 21.7%, p < 0.05. On the contrary, there were more obese patients (BMI > 30 kg/m2) among women who had never taken HRT: 56.5 versus 13% (p < 0.05). Among those who had never taken HRT, 21 (91.3%) patients had an abdominal type of fat tissue distribution (WC/TB > 0.8). Among women who took Femoston 2/10 for two years, this type of adipose tissue distribution was significantly less common - in 34.8% of cases (p < 0.05). WC in the group of women taking Femoston 2/10 was significantly less than in women who had never used HRT.
A retrospective assessment of changes in body weight after menopause or when taking HRT in groups of women examined showed that after taking Femoston 2/10 for two years, body weight did not increase in any patient, decreased by 3–9 kg in 8 (34.8%) and did not change in 15 (65.2%) women. In the group of patients who had never used HRT, 19 (82.6%) women reported an increase in body weight after menopause by 2–8 kg.
We also examined 20 postmenopausal women lasting more than a year (mean age: 52.3 years, mean postmenopausal duration: 2.1 years). All patients took the combination drug Femoston 1/5 (estradiol 1 mg, dydrogesterone 5 mg) continuously for 12 months. In addition to the standard anthropometric examination, these women initially underwent dual-energy x-ray absorptiometry (densitometry) after 6 and 12 months of therapy. It was used to determine bone mineral density, mass and percentage of fat.
At baseline, all 20 women were found to be overweight or obese, and all of them had an abdominal type of fat tissue distribution (WC/TB ≥ 0.8). Before the start of therapy, the average body weight in the group of patients was 82.3 kg, BMI – 29.1 kg/m2, WC/TB – 0.82, WC – 82.1 cm. Dynamics of anthropometric parameters during treatment with Femoston 1/5 indicated a decrease in all assessed parameters. Thus, after 6 months of therapy, body weight decreased on average in the group by 3.2 kg, and after 12 months by 3.8 kg (p = 0.05). Body weight did not change in 2 (10%) women, and WC in these patients decreased by 2.5 and 2.0 cm, but TB did not change. In the group as a whole, BMI decreased after 12 months of treatment from 29.1 to 28.5 kg/m2 (p = 0.05). WC decreased after 6 months by 3.5 cm, after 12 months – by 4.3 cm (p < 0.05).
Using densitometry, it was found that the total mass of adipose tissue decreased after 12 months by an average of 3.5 kg (range -2.7 to -3.7 kg; p < 0.05).
Thus, our data indicate that against the background of HRT in peri- and postmenopausal women, body weight and the amount of adipose tissue in the abdominal-visceral region decrease. Taking into account the known data on the mechanisms of the influence of estrogens and progesterone on adipose tissue, it can be assumed that the redistribution of adipose tissue is largely due to the influence of a deficiency of sex hormones than to age-related processes. The changes we recorded in body weight and the degree of abdominal obesity in menopausal women can be considered as clinical confirmation of the results of experimental studies on the participation of sex hormones in the regulation of metabolic processes in adipose tissue.
It is obvious that HRT does not affect caloric intake and physical activity. Therefore, important components of therapy are well-known behavioral methods for treating obesity: a low-calorie diet and regular physical activity.
Reasons for women
There are many reasons why a hormonal belly can appear in women. It is important to understand that this is a rather dangerous condition that requires prompt treatment. This means that an enlarged belly due to hormonal imbalance is not the worst thing. It is much worse if problems begin with the functioning of internal organs, pain and other pathological symptoms appear. The main reasons for the appearance of fat in the waist area are as follows.
- Hypothyroidism is a pathology of the thyroid gland in which the required amount of hormones is not produced. Metabolic processes are disrupted, causing body weight to increase.
- Tumor processes in the pancreas. Due to their appearance, there is an increased production of insulin, which reduces blood sugar levels. At the same time, a person feels severe hunger, and eating high-calorie foods during this period leads to weight gain.
- Tumor processes in the adrenal glands, ovaries, some infections and disruption of the functioning of the nervous system lead to changes in the hormonal balance of the body.
- Disturbances in the functioning of the hypothalamus and pituitary gland. These areas of the brain are responsible for the production of active substances that affect metabolic processes in the body. Due to the lack of these substances, metabolism is disrupted, which leads to the appearance of fat reserves.
- Changes in hormonal levels during pregnancy or after childbirth. The fat reserves that appear during pregnancy are not noticeable, because a woman’s figure is already changing. But after childbirth, it is impossible to quickly remove such a belly.
- Changes in hormonal levels after menopause. Here it will be more difficult to cope with fat deposits, but in some cases treatment helps.
Significant changes in your figure are a reason to think about your health
How to remove hormonal belly at home
After we figured out what it is - a hormonal belly, you need to decide what to do with it. There are several ways to remove hormonal belly fat in women on your own.
Diet
This method will help solve the problem in the early stages. But the diet is also prescribed in combination with taking pills if the hormonal belly is already quite large. Using a diet will not quickly get rid of the problem, but in general you will only do better for your body if you normalize your diet.
Only the attending physician has the right to advise diet or hormones
First, you need to increase the amount of complex carbohydrates in your diet. These are grains, cereals, vegetables and some fruits. It is advisable to eat all these products for breakfast or lunch.
You should avoid any sweets - fast carbohydrates primarily contribute to the deposition of fat in the abdominal area, says hudeem-bez-problem.ru.
Reduce the amount of animal fats and increase the amount of vegetable fats. Of the products containing animal fats, it is recommended to leave only sea fish and dairy products.
Try to eat food at the same time every day. In addition, do not forget to drink at least two liters of water to normalize metabolic processes.
Use of natural hormones
How to get rid of hormonal belly fat in women if diet no longer helps and you don’t want to take medications? After consulting with your doctor and passing all the necessary tests, you can include in your diet foods that contain natural hormones. Or, on the contrary, exclude from the diet those foods that negatively affect hormonal balance.
- Leptin will help you feel full faster, even with small amounts of food. This hormone will begin to be produced better in the body if you normalize your sleep.
- Ghrelin, when elevated, makes a person feel hungry. Ghrelin synthesis increases when fructose enters the stomach.
- Insulin, the deficiency of which causes excess weight accumulation. When you consume a lot of sugar, insulin is used up faster. A small amount of apple cider vinegar dissolved in water helps normalize insulin levels.
- Cortisol, the deficiency of which causes strong appetite. The amount of cortisol decreases with lack of sleep and rest.
- Estrogen, the deficiency of which reduces the rate of metabolic processes. The amount of the hormone increases from regular consumption of fresh vegetables, fruits and dairy products.
Massage
You can massage the problem area in a specialized salon or on your own. The positive effect of the procedure will be in any case, only in some places greater and in others less. But massage must be combined with treatment aimed at normalizing hormonal levels, otherwise there will be no positive result from the massage.
How massage helps for weight loss >>
Ab exercises
What exercises will help
How to remove a girl’s hormonal belly with exercises? To begin with, you should understand that training alone cannot cope with the problem. Even if you have pumped up abdominal muscles, they will hide under a layer of fat if fat deposits appear due to hormonal imbalances. Therefore, all trainers recommend going to the doctor if you exercise regularly and your waist is not getting thinner.
If you combine regular training with treatment, you will achieve the desired result much faster.
During the Lose-Weight-No-Problems classes, she advises to do exercises to strengthen the abs and abdominal wall, but do not forget about exercises for the rest of the muscles of the body. After all, the more calories you spend during a workout, the faster the excess fat will disappear.
At home, the well-known scissors, drawing and bicycle exercises, which do not require special equipment, are good for everyone. In all these exercises, a person, lying on his back, raises his legs up by about 45% and begins to perform various movements without lowering his legs. Squats with your legs spread hip-width apart will be effective. After finishing your workout, you should feel your abs slightly sore, especially when you laugh or otherwise tense your muscles.
Remember that to lose weight you need to use all the muscles and work the whole body
Men who want to get rid of abdominal problems should definitely include strength exercises in their training. This will lead to a decrease in cortisol levels and an increase in testosterone levels. Which will help reduce appetite and quickly return to your previous shape.